COVID-19 restrictions limit dental visits to all but emergency care, which means that millions of patients are overdue to have their teeth cleaned.
And because preventive services are not critical in the short term, all dental hygienists affected by the shut down are now out of work. However, the jobs will come back. What’s more concerning is the damage that may be happening in our patients’ mouths without a little extra intervention.
A few years back, do you remember the guidance that hygienists and dentists shared with you whenever we discovered that blue plastic bits were getting stuck under our patients’ gums? That was the dental community banding together here to get the word out, and we were able to convince manufacturers to stop adding plastic to toothpaste.
We’re coming together again in the same place, this time to pack a few personal items into your phone, tablet, computer, or whatever you’re looking at right now. This is the delivery we’re shipping to our patients, to take care of you with our thoughts and our hearts, and to share our best tips to make sure that you’re as healthy as possible at your next dental visit.
You want me to put my toothbrush where?
Care Package Item #1: Brushing your teeth with your other hand for the first minute, then switching hands.

This is all about about getting re-introduced to friction and tapping into novelty to help you learn something about yourself. When you pick up your toothbrush, you normally do so with your dominant hand. Instead (and this is THE KEY) you will grab your toothbrush with your other hand. If you’re a righty, then put your toothbrush in your left hand. Add toothpaste if you’d like and start brushing. Pay attention to how your gums feel when the bristles touch them. Most people don’t realize this, but they avoid touching their gums properly when they brush.
If it hurts to brush with your non-dominant hand, this is a sign that your toothbrush may be too hard, because soft brushes should feel pretty normal at this point. Keep brushing with your other hand, all around, then try to make the bristle contact feel the same when you place the toothbrush back in your dominant hand. It was surprising the first time I did this, because I realized I didn’t brush as well in areas that I thought I did. Even though I’m a hygienist, this helped me uncover weaknesses in my own brushing technique. Just watch out how you spit, because your other hand may now be in your trajectory field and end up all slobbery.
What’s your best flossing hack?
Care Package Item #2: Curved 3D flossers

As products go, this is a very specific one! There’s no substitute for using a string under your gumline, and if you have a tool that angles the string perfectly every time, you’re more likely to floss frequently and effectively. Make sure that your flosser is curved in 3D, not just flat:

With your 3D curved flosser, click the string between each place that your teeth make contact and floss deep under the gum tissue, once for each side, just like this:
I’ve demonstrated this on a front tooth for convenience, but the payoff of using these 3D flossers is when you get to really tight spaces in the back. My favorite brand is the Dentek Complete Clean Back Teeth, but there are others. Just make sure you purchase the curved flossers and not the flat ones.
Whoa, what should I do about blood or braces?
Care Package Item # 3: Soft Toothpicks
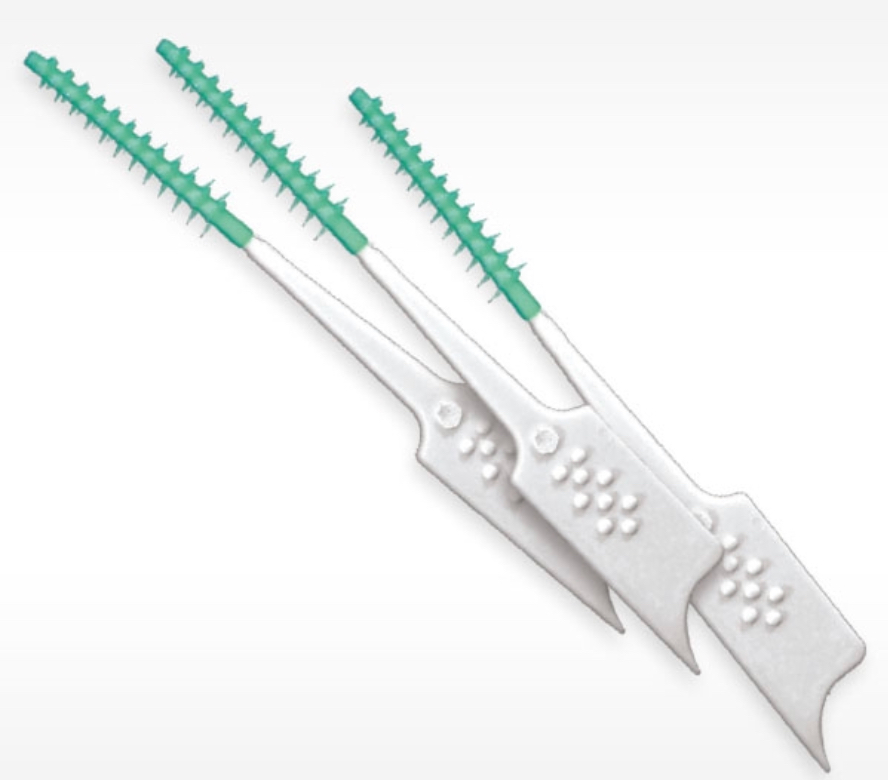
If you’re bleeding when you brush or floss, or can’t clean effectively with either of those items because of metalwork in your mouth, you’ve got to dig deeper. Remember, the stuff you’re trying to clean off of your teeth isn’t necessarily food; instead it grew there like a layer of pond scum, so you need to disrupt it as often as you clean your armpits.
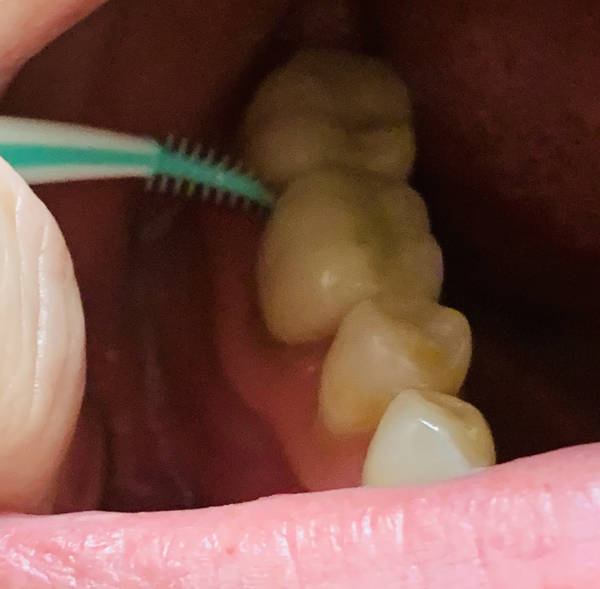
Try gently poking deep into the areas that tend to bleed easily, like the gum pockets you’ve been told you have (that’s mine up there), or create some friction up in the gumline next to an orthodontic bracket or retainer wire. As long as you don’t feel any pain, you should be able to sweep away the germs that contribute to tooth loss with interdental cleaners like the Gum brand Soft Pick.
No toothpaste? Are you kidding?
Care Package Item #4: Brushing without toothpaste first
Hear me out – I am addicted to the feeling of mouth freshness. This is what the detergent industry has turned me into, a little mint whore. However, the foamy nature of toothpaste tends to obscure exactly what it is that I’m brushing, so periodically, I’ll spend the first 30 seconds of my brushing ritual without toothpaste. After that, it’s like an exciting reward, a delayed gratification of sorts. Hey, when you have to stay isolated from the rest of society for a while, the little joys start adding up.
Will overeating contribute to cavities?
Care Package Item #5: Swishing with water all day

A side effect of sheltering in place is boredom eating, which means excess carbohydrates fermenting in our mouths leading to a rapid increase in dental decay. To combat this, each time you eat, be sure to swish with a mouthful of water immediately afterwards. This will help you rinse away excess particles and acid. Remember – dentists are discouraged from filling cavities at the moment – they are more likely to be put in a position to pull an otherwise good tooth to get you out of immediate pain and danger of having a life-threatening dental abscess.
We’ve shared this article with you because we’re worried! Priorities are going to change in the coming months, and our careers are built on the foundation that patients should be able to keep their teeth for a lifetime with minimal professional care. Stay healthy, friends, and we look forward to seeing you back in the office as soon as possible.

Trish Walraven, RDH BS is a dental hygienist in the Dallas/Fort Worth area who is sad for so many of her colleagues that have lost their livelihoods. She would like to inspire her fellow hygienists and dentists to feel brave enough to share their concerns and best home care ideas so that we can begin the work of reconnection.